The results of many interesting trials were presented at the TCT conference 2018 held in San Diego, California.
The COAPT trial
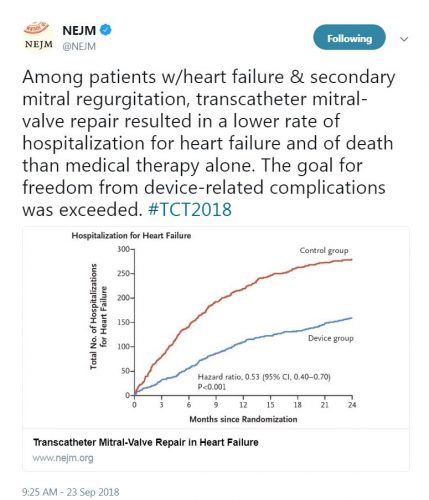
This prospective, multicenter, open-label, randomized trial aimed to assess the use of transcatheter mitral valve repair in Heart Failure (HF) patients with mitral regurgitation due to left ventricular dysfunction. A total of 614 patients were randomized to either receive guideline-directed medical therapy (GDMT) alone or GDMT in addition to transcatheter mitral-valve repair. The primary efficacy endpoint was all hospitalizations due to heart failure at 24 months of follow-up, while the primary safety endpoint was freedom from device-related complications at 12 months of follow-up.
The study showed that the device group had a lower risk of hospitalization for HF (HR 0.53, 95% CI 0.4-0.7) and all-cause mortality (HR 0.62, 95% CI 0.46-0.82) at 24 months. Moreover, the rate of freedom from device complications at 12 months was 96.6% which exceeded the prespecified safety threshold.

“In this trial, device-based treatment resulted in a significantly lower rate of hospitalization for heart failure, lower mortality, and better quality of life and functional capacity within 24 months of follow-up than medical therapy alone. In addition, the rate of freedom from device-related complications with transcatheter mitral-valve repair exceeded a prespecified objective performance goal.”- Dr. Gregg W. Stone, M.D.
The ULTIMATE trial
This multicenter, randomized trial compared the outcomes of Intravascular Ultrasound (IVUS)-guided drug-eluting stent (DES) implantation to angiography-guided DES implantation. A total of 1448 patients were randomized to receive either IVUS guidance or angiography guidance during DES implantation. The primary endpoint was target vessel failure at 12 months defined as cardiac death, target vessel myocardial infarction, and clinically-driven target vessel revascularization.
Patients with IVUS-guided DES implantation had lower target vessel failure compared to patients with angiography-guided DES implantation (HR 0.53, 95% CI 0.312-0.901). The authors concluded that the use of IVUS-guided DES implantation has significantly improved the clinical outcomes.

“In the present multicenter randomized trial, IVUS-guided DES implantation in all-comers resulted in lower incidence of TVF at 12 months, compared with angiography guidance, particularly for patients who had an IVUS-defined optimal procedure.”- Dr. Junjie Zhang, M.D.
The FAST-FFR trial
This international, multicenter, prospective trial aimed to assess the accuracy of fractional flow reserve derived from coronary angiography (FFRangio) compared to FFR measured with pressure wire. Patients with suspected coronary disease had coronary angiography and the FFR was measured using a pressure wire in the vessels having lesions. After blinding of the FFR results, operators calculated the FFRangio using proprietary software. The co-primary endpoints were the sensitivity and specificity of the dichotomously scored FFRangio for predicting pressure wire-derived FFR using a cutoff value of 0.80. The study was powered to meet prespecified performance goals for sensitivity and specificity.
The sensitivity and specificity of FFRangio per vessel was 94% and 91% respectively. Moreover, the diagnostic accuracy of FFRangio was 92% and the device success rate for FFRangio was 99%.

“I think FFRangio will replace a pressure wire, but we obviously need more data. The next step will be to do a clinical outcomes study comparing FFRangio guided strategy randomized to patients in an invasive pressure wire derived strategy and demonstrate the outcomes.”- Dr. William Fearon, M.D.
The ABSORB IV trial
This randomized, active-controlled, multicenter trial compared the clinical outcomes of bioresorbable vascular scaffolds (BVS) to those of metallic drug-eluting stents (DES). The study recruited 2604 patients from 147 centers in 5 countries. The patients were randomized and assigned to receive either polymeric everolimus-eluting BVS or cobalt-chromium everolimus-eluting stents. The patients were observed at 30 days for the occurrence of the primary endpoint of target lesion failure (cardiac death, target vessel myocardial infarction, and ischemia-driven target vessel revascularization).
At 30 days, target lesion failure occurred in 5.0% and 3.7% in patients assigned to BVS and Xience respectively (Difference 1.3%, upper 97.5% confidence limit 2.89; one-sided p non-inferiority=0.0244). At 1 year, target lesion failure occurred in 7.8% of patients assigned to BVS and 6.4% patients assigned to EES (difference 1.4%, upper 97.5% confidence limit 3.4; one-sided p non-inferiority=0.0006). The authors concluded that Absorb BVS was non-inferior to cobalt-chromium-based Xience DES up to 1 year for cardiovascular outcomes in fairly simple lesion types.

“It was a very, very interesting study. Better technique, better patient selection, and appropriate types of lesions do bring down this difference and I honestly believe that now all we need is a better device that will hopefully eliminate most or all of these early first 3 year differences, potentially allowing the long-term benefits of ABSORB to emerge.”- Dr. Gregg Stone, M.D.
The ReCre8 trial
This randomized, prospective, multicenter, physician-initiated trial aimed to compare the clinical safety and efficacy of Polymer-free amphilimus-eluting stents (PF-AES) to permanent-polymer zotarolimus-eluting stents (PP-ZES). A total of 1502 patients were assigned to receive either PP-ZES or PF-AES after stratification for diabetes and troponin status. The patients were followed for 12 months for the occurrence of the primary endpoint of target lesion failure (cardiac death, target vessel myocardial infarction, and target vessel revascularization).
The primary endpoint occurred in 5.6% and 6∙2% of the patients receiving PP-ZES and PF-AES respectively. The investigators found that PF-AES were clinically non-inferior to PP-ZES (risk difference 0∙5%, upper limit one-sided 95% confidence interval 2∙6%, p non-inferiority 0∙0086).

“While PF-AES is non-inferior to PP-ZES in regard to the primary endpoint of target-lesion failure at 12 months follow-up, a future dedicated trial on PF-AES in diabetic patients is required in order to explore the efficacy of this novel drug-eluting technology in this specific subgroup.”- Dr. Pieter Stella, M.D.
Social media feedback





Leave a Reply
You must be logged in to post a comment.